Because electrocommunication signals are strongly regulated by gonadal hormones and are controlled by a simple neural circuit, they serve as an excellent model for understanding how hormones act on the brain to produce sex differences in behavior. Moreover, because sex differences and hormonal regulation of EODs vary across species, we can also study how neuroendocrine mechanisms evolve to produce species diversity in sexually dimorphic behavior.
We have taken advantage of the fact that EOD frequency is controlled by the medullary pacemaker nucleus to examine how variation in gene expression in the brain is linked to variation in the hormonal regulation and sexual dimorphism of behavior.
Specifically, we have:
(1) Used transcriptomics to identify genes that are expressed in the pacemaker nucleus and genes whose expression varies across sexes and/or species (Smith et al., 2018).
(2) Used quantitative PCR to study how expression of genes whose products mediate gonadal steroid hormone actions vary across species and populations in that differ in the sexual dimorphism of EOD frequency (Proffitt and Smith, 2024).
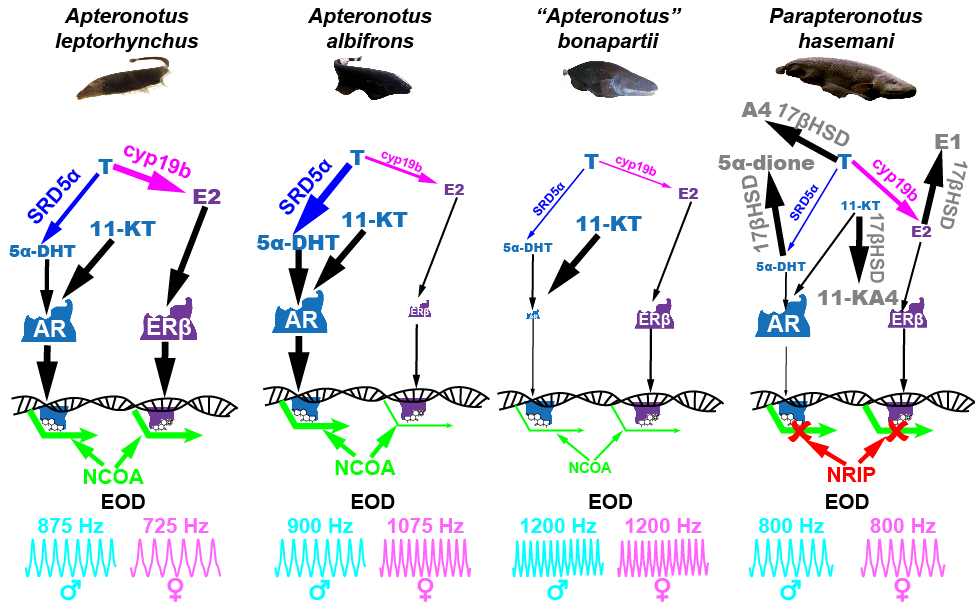
(3) Measured expression of genes for steroid hormone receptors and metabolizing enzymes in electrosensory brain regions that allow electric fish to perceive each other’s electrocommunication signals (Freiler et al., 2024).
(4) Studied the evolution of the sequence and function of androgen receptors across electric fish species that differ in how androgens regulate electrocommunication signals (Proffitt and Smith 2023).

Species differences in structure and function of androgen receptors may influence how androgens regulate sex differences in EOD frequency. Like many teleost fishes, South American knifefishes have two genes for nuclear androgen receptors (AR-alpha and AR-beta). Both ARs are expressed in the pacemaker nucleus in A. albifrons and A. leptorhynchus, and AR-alpha is expressed at higher levels than AR-beta. These two species differ in the direction of sex differences and androgenic regulation of EOD frequency – EOD frequency is lower in males and reduced by androgens in A. albifrons, but is higher in males and increased by androgens in A. leptorhynchus. The structure of AR-alpha differs between these species – several mutations in the ligand binding domain of AR-alpha in A. leptorhynchus render this AR unable to bind to androgens, whereas AR-alpha in A. albifrons retains its ability to bind androgens. We hypothesize that the loss of AR-alpha binding in A. leptorhynchus may contribute to the reversal in the direction of sex differences in EODf. Specifically, we hypothesize that AR-alpha and AR-beta have opposing effects on EODf, with AR-alpha lowering EOD frequency and AR-beta raising EODf. In A. albifrons, greater expression of AR-alpha in the pacemaker nucleus results in a net effect of androgens lowering EOD frequency. In A. leptorhynchus, AR-alpha is unable to bind to androgens, and the effects of androgens may be mediated primarily by AR-beta, which we hypothesize raises EOD frequency. Adapted from Proffitt and Smith 2023.
References
Freiler, M.K., Deckard, M.R., Proffitt, M.R., and Smith, G.T. (2024) Differential expression of steroid-related genes across electrosensory brain regions in two sexually dimorphic species of electric knifefish. Gen. Comp. Endocrinol. 355:114549.
Proffitt, M.R., Liu, X., Ortlund, E.A., and Smith, G.T. (2023) Evolution of androgen receptors contributes to species variation in androgenic regulation of communication signals in electric fishes. Mol. Cell. Endocrinol. 578:112068.
Proffitt, M.R. and Smith, G.T. (2024) Species variation in steroid hormone-related gene expression contributes to species diversity in sexually dimorphic communication in electric fishes. Horm. Behav. 164:105576.
Smith, G.T., Proffitt, M.R., Smith, A.R., and Rusch, D.B. (2018) Genes linked to species diversity in a sexually dimorphic communication signal in electric fish. J. Comp. Physiol. A 204:93-112.
