The Electromotor System is a Model for Motor Pattern Generation. The electric organ discharge (EOD) and the regular firing of the neurons that control it are among the fastest and most precise biological oscillations. In apteronotid electric fish, EOD frequency can approach 2000 Hz. The firing rates of pacemaker neurons and electromotor neurons correspond directly to EOD frequency. These neurons thus produce action potentials synchronously at rates of up to 2000 times per second, which makes them the most rapidly firing neurons in any animal. One of the goals of our research is to understand the cellular mechanisms that underlie these rapid and precise motor patterns and to understand how variation in neuronal properties among individuals and between sexes and species is related to behavioral diversity.
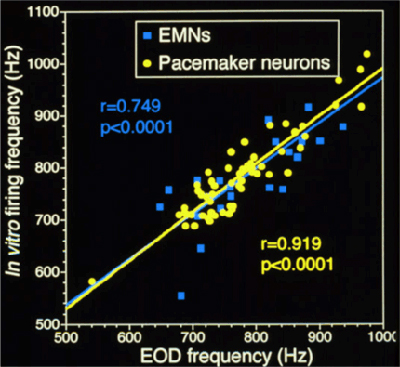
Pacemaker and electromotor neurons continue to fire spontaneously in vitro. Studying the cellular mechanisms of high-frequency motor rhythms is facilitated by the simplicity of the neural circuits that control the EOD and by the fact that these neurons maintain in vivo-like activity when recorded in vitro. (click here for background information on the electromotor circuit). The pacemaker nucleus and electromotor neurons can be removed from the animal and kept alive in artificial cerebrospinal fluid. These neurons maintain spontaneous firing rates that approximate the in vivo EOD frequency of the fish from which they were taken (see figure below). This allows us to use electrophysiological techniques to study these neurons under controlled, in vitro, conditions, but still be able to understand how variation in the activity of these neurons between individuals, sexes, or species is related to the in vivo electrical behavior of the fish.
We use three approaches to study mechanisms of high frequency motor pattern generation in pacemaker and electromotor neurons:
Experiments with Channel-Blocking Drugs to Identify Ionic Currents that Control Spontaneous Firing: Because both pacemaker neurons and electromotor neurons continue to fire in vitro at frequencies very similar to those in vivo, we are able to record their spontaneous firing patterns to study how neuronal excitability influences behavior. We can then apply drugs that specifically block particular classes of ion channels to identify the ionic currents that generate the high-frequency firing patterns that control the EOD rhythm. We have used this technique to study ionic currents that control high-frequency firing in pacemaker neurons (Smith and Zakon 2000) and electromotor neurons (Smith 2006). These studies revealed that sodium and potassium currents are critical for generating and regulating the motor rhythm for the EOD: (1) fast, transient sodium currents rapidly generate the depolarizing phase of action potentials; (2) persistent sodium currents regulate firing rates and provide tonic depolarization to maintain high-frequency firing; and (3) potassium currents carried by Kv1-like channels contribute to the repolarization of the action potential and regulate firing rates. We have also used immunohistochemistry to show that several different Kv1 potassium channels are expressed in the pacemaker nucleus and in the axons of electromotor neurons (Smith, et al. 2006).
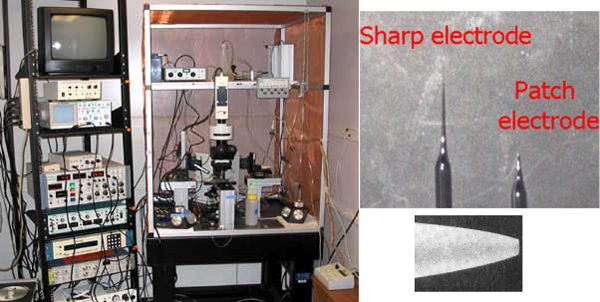
Voltage-clamp Experiments to Characterize the Biophysical Properties of Ionic Currents in Pacemaker and Electromotor Neurons: We use a microscope with Nomarski optics and an infrared-sensitive video camera to visually guide fire-polished glass micropipettes onto the membranes of pacemaker or electromotor neurons. We can then use voltage-clamp recordings to measure biophysical properties of the ionic currents that contribute to the spontaneous, high-frequency firing of these neurons. These studies allow us to examine the mechanisms of sex and individual differences in behavior from the level of single cells to entire organisms, and to study how these mechanisms evolve to produce different behaviors across species.
Characterizing the expression of ion channel genes in the pacemaker nucleus. We use transcriptomic approaches and quantitative PCR to measure the expression of genes for ion channels in the pacemaker nucleus. This approach allows us to examine how ion channels in the pacemaker nucleus vary across sexes and species with different EOD frequencies (and thus different levels of activity in pacemaker neurons).
References
Meyer, J.H. 1984. Steroid influences upon discharge frequencies of intact and isolated pacemaker of weakly electric fish. J. Comp. Physiol. A 154:659-668.
Schaefer, J.E. and Zakon, H.H. 1997. Opposing actions of androgen and estrogen on in vitro firing frequecny of neuronal oscillators in the electromotor system. J. Neurosci. 16:2860-2868.
Smith, G.T. and Zakon, H.H. 2000. Pharmacological characterization of ionic currents that regulate the pacemaker rhythm in a weakly electric fish. J. Neurobiol. 42:270-286.
Smith, G.T. 2006. Pharamacological characterization of ionic currents that regulate high-frequency spontaneous activity of electromotor neurons in the weakly electric fish, Apteronotus leptorhynchus. J. Neurobiol. 68:1-18.
Smith, G.T., Unguez, G.A., and Weber, C. 2006. Distribution of Kv1-like potassium channels in the electromotor and electrosensory systems of a weakly electric fish. J. Neurobiol. 66:1011-1031.
